Using the Safety-EDC Connection, organizations with both a Safety Vault and a Clinical Data EDC Vault can exchange data about serious adverse events (SAEs) discovered by study sites from your EDC Vault to your Safety Vault for Case processing, helping you to avoid delays and compliance issues. Site Investigators can enter and update Safety Case Initiation Event forms in your EDC Vault and Vault automatically transfers the SAE data, along with the applicable subject and Case information, to your Safety Vault. Case Processors and Medical Reviewers can then triage, review, and process the Case according to the submission requirements of the relevant regional health authorities for a particular study. This allows your organization to minimize the risk of data entry mistakes and respond to SAEs in the designated time period, avoiding delays and compliance problems.
When a site user creates an SAE in their EDC Vault, Vault generates an initial Inbox Item in the Safety Vault in the New lifecycle state. You can review the new Inbox Item and promote it to an Initial Case or Follow-up Case, when applicable. If your Admin has configured automated Case promotion and a site user later updates that SAE in their EDC Vault, Vault generates a new Inbox Item in the Marked as Follow-up lifecycle state in the Safety Vault and links it to the appropriate Case, which you can review and optionally merge into the existing Case. For Cases created or updated based on SAE data from the connection, you can review and adjust all relevant subject information.
Note: Depending on your Admin’s configuration, object, field, and section labels, lifecycle states, and workflows may differ from the general information on this page. Refer to your organization’s business processes for guidance.
How the Safety-EDC Connection Works
The Safety-EDC Connection creates new records and updates existing records across Vaults in the following situations:
- When a site user creates or updates SAE subject data in an EDC Vault, Vault creates or updates the corresponding Inbox Item, Case, and CDMS subject information records in the Safety Vault based on Admin-defined field mapping.
- When a site user creates or updates SAE case data in an EDC Vault, Vault creates an Inbox Item in the Safety Vault.
- When configured by your Admin, Cases created and updated in the Safety Vault based on data received from the Safety-EDC Connection may be automatically promoted.
- If a Case does not yet exist in the Safety Vault, the Inbox Item is created in New state. If your Admin has configured automated Case promotion and a Case already exists in the Safety Vault, the Inbox Item is created in Marked as Follow-up state.
- When a user clears the SAE by clicking the Mark as Blank button, the generated Inbox Item has an EDC Reason value of “Form cleared by the EDC user, representing a void or nullification scenario”. If a relevant Case exists, it updates to Marked as Follow-up state.
- When a user downgrades an adverse event from serious to not serious, the change is reflected on the Case Compare page, and the Case updates to Marked as Follow-up state.
- When a user deletes subject data related to an SAE in an EDC Vault, Vault marks the corresponding CDMS subject information records in the Safety Vault as deleted and creates an Inbox Item for each nullified Case associated with the deleted subject. The EDC Reason value on these Inbox Items is “Subject Deleted on Vault EDC”. Vault does not delete the associated Inbox Item or Case when deleting subject information records.
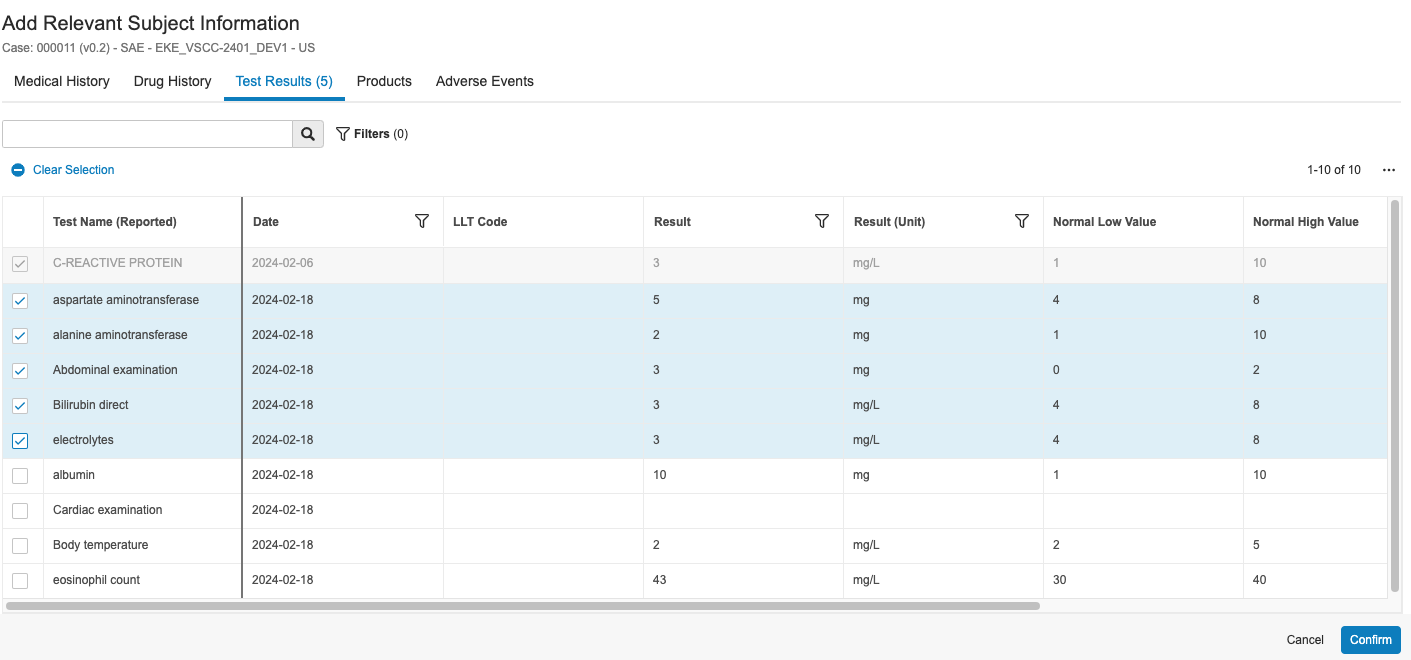
- Any EDC Local Lab results integrated into the EDC subject casebook in the EDC Vault transfer to the Inbox Item and corresponding lab section in the Case record in your Safety Vault. You can also review the data on the Test Results tab of the Add Relevant Subject Information dialog. When a user populates any Analyte value for a specific lab panel in EDC, all available Analytes for the panel display in your Safety Vault, including any Analytes without values.
About Record Creation & Updates
When creating a record through the connection, Vault automatically populates certain standard fields based on your Admin’s mapping. Vault also populates the Link (link__sys) field on the target record with the source record’s Global ID (global_id__sys), which lets Vault know which records to update in the target Vault when data is updated in the source Vault.
When Vault creates a Case in your Safety Vault through the connection, Vault populates fields on the source Safety Case record to reference the name and ID of the target record.
Vault automatically populates the Study and Study Product fields on generated Inbox Items if there are matching records in your Safety Vault. If no matches are found, the Inbox Item will be in Missing Information state. You can then manually populate the blank fields.
Vault creates all new records in their lifecycles’ initial states except generated Inbox Items, which may be in Missing Information state if required fields are blank.
CDMS Subject Information Records
Vault transfers clinical subject data from an EDC Vault to CDMS subject information records in your Safety Vault, which includes a parent CDMS Subject Information record and the following child records:
- CDMS Subject Adverse Event
- CDMS Subject Case Product
- CDMS Subject Case Product Dosage
- CDMS Subject Cause of Death
- CDMS Subject Drug History
- CDMS Subject Event Product Assessment
- CDMS Subject Height History
- CDMS Subject Last Menstruations
- CDMS Subject Links
- CDMS Subject Medical History
- CDMS Subject Pregnancy Information
- CDMS Subject Test Results
- CDMS Subject Weight History
Reviewing & Adding Relevant Subject Information
For SAEs transferred from an EDC Vault, you can run the Add Relevant Subject Information action on the applicable Case to review all relevant subject data, including medical and drug history, test results, and adverse events. You can then select which relevant subject data you want to add to the Case.
Note: This action is only available on Cases with subject data received from an EDC Vault that your Access Group allows you to view.
To add data based on subject information received from your EDC Vault and automatically create and relate the applicable records:
- Navigate to the applicable Case record.
- From the All Actions menu, select Add Relevant Subject Information.
- In the Add Relevant Subject Information dialog, select the applicable items you want to add to the Case.
- Optional: Use the search bar and filters to help find the items you want to add. Search results are case-sensitive. Any column that references a raw object will sort all values starting with capital letters before all values starting with lowercase letters.
- Optional: Click the Actions menu and select Edit Columns to select which columns to display in the dialog.
- Click the tabs to navigate between the options:
- Medical History
- Drug History
- Test Results
- Products: Vault automatically assigns the Drug Role of “Concomitant” to all Products you add. You cannot remove the study’s Products in this dialog.
- Adverse Events: You can only add Adverse Events not already linked to another SAE.
- When you’ve made your selections, click Confirm.
Vault automatically adds the applicable records to the appropriate sections in the Case record based on the items you selected in the dialog.
Note: You cannot remove Products in the Add Relevant Subject Information dialog, but if you manually delete a Study Product from a Case record’s Details page and then run the Add Relevant Subject Information action, you can re-add the related Product in the dialog. If you do this, Vault automatically assigns the Product Type of “Company Product” and the Drug Role of “Concomitant”, regardless of the original values.
Reviewing Subject Information
When configured by your Admin, you can review subject-specific information on a CDMS Subject Information record’s details page using the following layouts on any record generated after running the Add Relevant Subject Information action on a Case:
- Triage: Overview of all pending Inbox Items and any additional Cases for the subject.
- Subject Information: Comprehensive view of the subject, allowing you to identify and review any unexpected data.